Cellino successfully manufactures and delivers autologous iPSC lines from four donors to Matricelf for groundbreaking neural tissue engineering
Cellino and Matricelf (TASE: MTLF) announced today a collaboration to accelerate the global biomanufacturing of personalized spinal cord injury treatments using Cellino’s Nebula™ technology combined with Matricelf’s breakthrough regenerative approach. This collaboration combines Cellino’s automated iPSC manufacturing with Matricelf’s double autologous 3D differentiation process, paving the way for scalable, patient-specific regenerative therapies.
This press release features multimedia. View the full release here: https://www.businesswire.com/news/home/20250311183832/en/
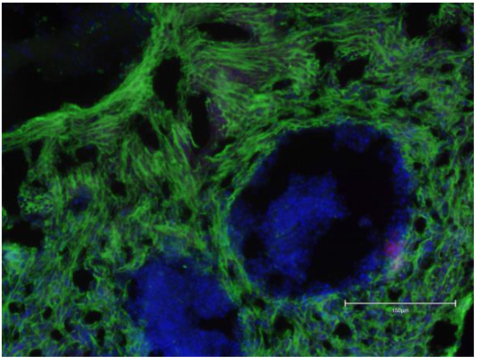
Image caption: Cellino generated iPSC transformed by Matricelf into functional neural tissue (Photo: Business Wire)
Nebula™, Cellino’s proprietary closed-cassette biomanufacturing system, is designed to produce high-quality induced pluripotent stem cells (iPSCs) at scale with unparalleled consistency and sterility. This innovation allows for on-demand, contamination-free iPSC production, bringing regenerative medicine closer to real-world clinical application.
As a key milestone, Cellino has successfully manufactured and delivered autologous iPSC lines from four donors to Matricelf, a biotech company developing regenerative therapies for spinal cord injury. Matricelf’s approach integrates an extracellular matrix-based hydrogel derived from the patient’s own omentum, facilitating precise cell differentiation and tissue formation while eliminating the need for immunosuppression.
Matricelf has now transformed Cellino-generated iPSCs into functional neural tissues, demonstrating synchronized electrical activity—a hallmark of functional neural networks. Rigorous industry-standard assays confirmed key neural characteristics, including genetic and protein neural marker expression of engineered tissues with Cellino’s iPSCs, with results comparable to Matricelf’s own iPSC-derived tissues.
With the goal of redefining treatment paradigms for spinal cord injury, Matricelf plans to file an Investigational New Drug (IND) application next year to bring its autologous iPSC-derived therapy into clinical trials. This transformative approach addresses an urgent unmet need, potentially restoring function for millions of patients worldwide.
“Collaboration on this international scale is a vital step toward our vision of a fully autologous regenerative therapy for spinal cord injury,” said Gil Hakim, CEO of Matricelf. “Matricelf’s innovative platform, in combination with Cellino’s Nebula™ technology, is setting new standards in scalable patient-specific tissue engineering, bringing us closer to real-world applications of regenerative medicine.”
“The ability to produce high-quality, patient-specific iPSCs at scale is poised to revolutionize regenerative medicine,” said Marinna Madrid, Ph.D., Co-Founder and Chief Product Officer, Cellino. “Our collaboration with Matricelf exemplifies how international collaboration can accelerate innovation and expand access to life-changing therapies.”
This collaboration highlights both companies' commitment to harnessing advanced technologies - ranging from AI-driven automation and high-throughput biomanufacturing to pioneering sophisticated neural tissue engineering to accelerate the commercialization of next-generation regenerative therapies for neurological diseases worldwide.
About Cellino
Cellino is a leader in advanced biomanufacturing technology, committed to making personalized cell, tissue, and organ replacements a reality for patients around the world. Learn more at cellinobio.com and follow us on LinkedIn and X.
View source version on businesswire.com: https://www.businesswire.com/news/home/20250311183832/en/
Contacts
Media Contact
Kimberly Ha
KKH Advisors
917-291-5744
kimberly.ha@kkhadvisors.com




