- Identifying GP2 specific T cell immune response at baseline prior to treatment with GP2 has potential to predict breast cancer recurrence risk and timing of recurrence
- A positive baseline immune response to GP2 in 22.8% of 145 patients suggests an existing immune response to GP2 associated with residual disease, impending recurrence, or prior treatments
- DNA sequencing of relevant GP2 specific T cells at baseline and during GP2 treatment could lead to potential CAR-T cell drug candidates
- Data further validates 0% metastatic breast cancer recurrence mechanism and reaffirms that GP2 is a natural antigen that should be the target of peptide and T cell based platform technologies
Greenwich LifeSciences, Inc. (Nasdaq: GLSI) (the “Company”), a clinical-stage biopharmaceutical company focused on the development of GP2, an immunotherapy to prevent breast cancer recurrences in patients who have previously undergone surgery, today announced the publication of a poster for the GP2 Phase IIb clinical trial at the San Antonio Breast Cancer Symposium 2021 (SABCS). The CEO of Greenwich LifeSciences, Snehal Patel, recorded an audio track providing an overview. The abstract can be viewed at the bottom of this press release. The full poster with figures, tables, and audio can be accessed or downloaded from the Company’s website here.
This press release features multimedia. View the full release here: https://www.businesswire.com/news/home/20211209005502/en/
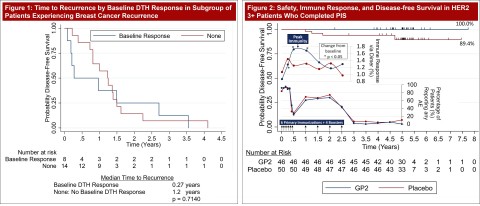
Figures 1-2: Summary of 5 year GP2 Data Published to Date (Graphic: Business Wire)
Figure 1 is the new data published yesterday evening at SABCS, which shows that GP2 immune response at baseline could be a prognosticator of cancer recurrence. The Phase IIb clinical trial enrolled HER2 positive patients, who received a standard course of trastuzumab after surgery, and HER2 low patients, who did not receive trastuzumab after surgery. A Delayed-Type Hypersensitivity (DTH) reaction was used to assess baseline in vivo immune responses to GP2 in patients prior to exposure to GP2 treatment or placebo.
The poster data can be summarized as follows:
‒ It was observed that 22.8% or 33 patients of the 145 patients reacted to GP2 at baseline with a positive immune response, which is defined as an induration of 5 mm or greater in the baseline DTH test.
‒ Of the 33 patients who did have a positive baseline DTH immune response to GP2, 8 patients recurred, which is a recurrence rate of 24.2% over 5 years of follow up, with a median time to recurrence of 99 days (0.27 years).
‒ Of the 77.2% or 112 patients who did not have a positive DTH baseline immune response to GP2, 14 patients recurred, which is a recurrence rate of 12.5% over 5 years of follow up, with a median time to recurrence of 438 days (1.2 years).
Mr. Patel commented, “This new GP2 specific T cell data suggests that patients with a positive baseline immune response to GP2 recurred twice as fast and approximately 7 to 11 months sooner than those without a positive baseline immune response did. While this data is very promising, the number of recurrences are low, thus we need to further confirm these observations in the upcoming Phase III clinical trial to determine if they are statistically significant. To further diversify our pipeline, we plan to fully characterize GP2 specific T cells by sequencing the DNA of the T cells at baseline and after treatment with GP2 to assess how these T cells change over time and if they can be developed into CAR-T drug candidates. Expansion into GP2 specific CAR-T cells could potentially become another platform technology to complement GP2 peptide treatment in higher risk patients. We expect new T cell data from the Phase III trial to become available in 2022.”
Today is the one year anniversary of the Company’s SABCS 2020 poster, which became the basis for Figure 2. This figure summarizes the efficacy, immune response, and safety Phase IIb data presented over the past year. The Kaplan Meier analysis for HER2 positive patients treated with GP2 immunotherapy shows 100% disease free survival (0% breast cancer recurrences, p = 0.0338) following surgery and Herceptin treatment over median 5 years of follow-up. These patients completed the Primary Immunization Series (PIS) which led to peak immunity at 6 months. No serious adverse events attributable to GLSI-100 were observed. Figure 1 and Figure 2 summarize all of the 5 year GP2 data published to date.
SABCS Abstract P2-13-29:
Title: Analysis of GP2 immune response and relationship to recurrence in a prospective, randomized, placebo-controlled, single-blinded, multicenter, phase IIb study evaluating the reduction of recurrences using HER2/neu peptide GP2 (GLSI-100) vs. GM-CSF alone after adjuvant trastuzumab in HER2 positive women with operable breast cancer
Snehal S Patel, David B McWilliams, Mira S Patel, Christine T Fischette, Jaye Thompson and F Joseph Daugherty.
Greenwich LifeSciences, Stafford, TX
Background: Delayed type hypersensitivity (DTH) skin tests in the randomized, active-controlled, single-blinded, multicenter Phase IIb trial investigating GLSI-100 (GP2+GM-CSF) administered in the adjuvant setting to node-positive and high-risk node-negative breast cancer patients with tumors expressing any degree of HER2 (immuno-histochemistry [IHC] 1-3+) (NCT00524277) have been analyzed. The trial enrolled HLA-A*02 patients randomized to receive GLSI-100 versus GM-CSF alone. The trial's primary objective was to determine if treatment with GLSI-100, a HER2-derived peptide, reduces recurrence rates. Analyses for this trial showing GLSI-100 to be efficacious, safe and immunogenic have been previously reported by Patel et al. and Mittendorf et al.
Methods: Consented patients were randomized and scheduled to receive GLSI-100 (500 mcg GP2: 125 mcg GM-CSF) or control (GM-CSF only) via 6 intradermal injections every 3-4 weeks as part of the Primary Immunization Series (PIS) for the first 6 months and 4 booster intradermal injections every 6 months thereafter. Boosters were introduced during the trial, thus some patients did not receive all 4 boosters. DTH skin tests were assessed at baseline and after the 6th dose with the orthogonal mean of each skin reaction measured 48-72 hours after injection using the sensitive ballpoint-pen method.
Results: The study enrolled 180 patients across 16 clinical sites with both HER2 3+ positive and low HER2 expressors (1-2+). After 5 years of follow-up, the Kaplan-Meier estimated 5-year DFS rate in the 46 HER2 3+ patients treated with GLSI-100, if the patient completed the PIS, was 100% versus 89.4% (95% CI:76.2, 95.5%) in the 50 placebo patients treated with GM-CSF (p = 0.0338). GLSI-100 was shown to be well tolerated with no SAEs deemed related to study medication and elicited a potent immune response measured by local skin tests and immunological assays. Injection site reactions were common, occurring in almost 100% of patients treated with either GLSI-100 or GM-CSF alone. Previous publications have reported the increase in DTH response reported among patients after treatment with GLSI-100. However, it was of interest to understand the positive DTH responses to GP2 noted at baseline. 22.8% of patients reacted to GP2 at baseline with induration of 5mm or greater. In the subgroup of patients who later experienced a breast cancer recurrence, 36.4% (8/22) had such a baseline response. Analysis of the time to recurrence among those recurring found that the median time to recurrence was 0.6 years for those with a baseline response while those that did not have a positive baseline DTH response to GP2 took 1.2 years to recur.
Conclusions: This study demonstrated that GLSI-100 safely elicited a potent immune response as evidenced by increased DTH skin responses with treatment paired with improved disease-free survival. It is theorized that a positive baseline DTH skin test to GP2 may be evidence of an existing immune response to GP2 associated with residual disease, impending recurrence, or prior treatments. Further studies assessing if GP2 immune response is an important prognosticator of cancer disease state or recurrence are planned.
About SABCS
The 44th annual SABCS has grown to be the industry’s premier breast cancer conference for basic, translational, and clinical cancer research professionals. It is well-known for presenting the latest breast cancer data from all over the world. More than 7,500 health care professionals from more than 90 countries attend annually. Baylor College of Medicine became a joint sponsor of SABCS in 2005. The Cancer Therapy & Research Center at UT Health Science Center San Antonio and American Association for Cancer Research began collaborations with SABCS in 2007. For more information, please visit the conference website at: https://www.sabcs.org/
About FLAMINGO-01 and GLSI-100
The Phase III clinical trial will be called FLAMINGO-01 and the combination of GP2 + GM-CSF will be called GLSI-100. The Phase III trial is comprised of 2 blinded, randomized, placebo-controlled arms for approximately 500 HLA-A*02 patients and 1 open label arm of up to 100 patients for all other HLA types. An interim analysis has been designed to detect a hazard ratio of 0.3 in IDFS, where 28 events will be required. An interim analysis for superiority and futility will be conducted when at least half of those events, 14, have occurred. This sample size provides 80% power if the annual rate of events in placebo-treated subjects is 2.4% or greater. The trial is currently being registered on clinicaltrials.gov and the link and trial identifier will be published shortly. For future updates about FLAMINGO-01 please visit the Company’s clinical trial tab at https://greenwichlifesciences.com/clinical-trials/.
About Breast Cancer and HER2/neu Positivity
One in eight U.S. women will develop invasive breast cancer over her lifetime, with approximately 282,000 new breast cancer patients and 3.8 million breast cancer survivors in 2021. HER2/neu (human epidermal growth factor receptor 2) protein is a cell surface receptor protein that is expressed in a variety of common cancers, including in 75% of breast cancers at low (1+), intermediate (2+), and high (3+ or over-expressor) levels.
About Greenwich LifeSciences, Inc.
Greenwich LifeSciences is a clinical-stage biopharmaceutical company focused on the development of GP2, an immunotherapy to prevent breast cancer recurrences in patients who have previously undergone surgery. GP2 is a 9 amino acid transmembrane peptide of the HER2/neu protein. In a randomized, single-blinded, placebo-controlled, multi-center (16 sites led by MD Anderson Cancer Center) Phase IIb clinical trial, no recurrences were observed in the HER2/neu 3+ adjuvant setting after median 5 years of follow-up, if the patient received the 6 primary intradermal injections over the first 6 months (p = 0.0338). Of the 138 patients that have been treated with GLSI-100 to date over 4 clinical trials, treatment was well tolerated and no serious adverse events were observed related to the immunotherapy. Greenwich LifeSciences is planning to commence a Phase III clinical trial using a similar treatment regime as the Phase IIb clinical trial. For more information on Greenwich LifeSciences, please visit the Company’s website at www.greenwichlifesciences.com and follow the Company's Twitter at https://twitter.com/GreenwichLS.
About GP2 Immunotherapy Immune Response
As previously reported, GP2 immunotherapy generated GP2-specific immune responses, leading to no metastatic breast cancer recurrence in the HER2/neu 3+ population in the Phase IIb clinical trial, thus supporting GP2’s mechanism of action. Statistically significant peak immunity was reached after 6 months of GP2 treatment, as measured in both the Dimer Binding Assay and the DTH skin test. HER2/neu 3+ population immune response was similar to the HER2/neu 1-2+ population immune response, suggesting the potential to treat the HER2/neu 1-2+ population (including triple negative breast cancer) with GP2 immunotherapy in combination with trastuzumab (Herceptin) based products and other clinically active agents. The broad based immune response suggests the potential for GP2 to treat other HER2/neu 1-3+ expressing cancers. For more information on GP2 immune response and clinical data, please visit the Company’s clinical trial tab at https://greenwichlifesciences.com/clinical-trials/.
Forward-Looking Statement Disclaimer
Statements in this press release contain “forward-looking statements” that are subject to substantial risks and uncertainties. All statements, other than statements of historical fact, contained in this press release are forward-looking statements. Forward-looking statements contained in this press release may be identified by the use of words such as “anticipate,” “believe,” “contemplate,” “could,” “estimate,” “expect,” “intend,” “seek,” “may,” “might,” “plan,” “potential,” “predict,” “project,” “target,” “aim,” “should,” "will,” “would,” or the negative of these words or other similar expressions, although not all forward-looking statements contain these words. Forward-looking statements are based on Greenwich LifeSciences Inc.’s current expectations and are subject to inherent uncertainties, risks and assumptions that are difficult to predict, including statements regarding the intended use of net proceeds from the public offering; consequently, actual results may differ materially from those expressed or implied by such forward-looking statements. Further, certain forward-looking statements are based on assumptions as to future events that may not prove to be accurate. These and other risks and uncertainties are described more fully in the section titled “Risk Factors” in the final prospectus related to the public offering filed with the SEC. Forward-looking statements contained in this announcement are made as of this date, and Greenwich LifeSciences, Inc. undertakes no duty to update such information except as required under applicable law.
View source version on businesswire.com: https://www.businesswire.com/news/home/20211209005502/en/
GLSI Announces Presentation of 5 Year Data for GP2 Phase IIb Clinical Trial, Revealing Potential For New T Cell Platform Technology
Contacts
Company Contact
Snehal Patel
Investor Relations
Office: (832) 819-3232
Email: info@greenwichlifesciences.com
Investor & Public Relations Contact for Greenwich LifeSciences
Dave Gentry
RedChip Companies Inc.
Office: 1-800-RED CHIP (733 2447)
Cell: (407) 491-4498
Email: dave@redchip.com




