Represents the Company’s thirteenth product launch since inception in October 2023
Avenacy, a specialty pharmaceutical company focused on supplying critical injectable medications, today announced it has launched Metoclopramide Injection, USP in the United States as a therapeutic generic equivalent for Reglan® as approved by the U.S. Food and Drug Administration. Metoclopramide Injection, USP is indicated for the relief of symptoms associated with diabetic gastroparesis, the prevention of nausea and vomiting associated with emetogenic cancer chemotherapy, the prevention of postoperative nausea and vomiting, small bowel intubation, and radiological examination.
This press release features multimedia. View the full release here: https://www.businesswire.com/news/home/20240916846646/en/
(Photo: Business Wire)
“In the ten months since launching as a new specialty pharmaceutical company, Avenacy has made unprecedented progress in delivering critical injectable medications to the U.S. market at an industry-leading pace,” said Jeff Yordon, Co-Founder and CEO of Avenacy. “The launch of Metoclopramide, our thirteenth commercial product, is the latest testament to our positive momentum and growing product register. Following the recent and significant capital infusion from our new equity investor, we are well-positioned to continue our strong trajectory by doubling down on growth initiatives that will allow us to expand our partner network, scale our business, and strengthen our commercial portfolio.”
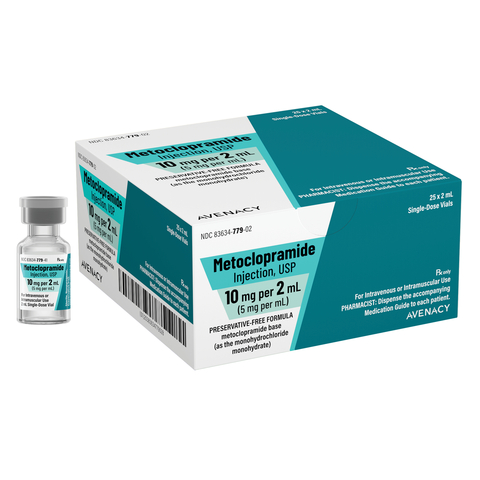
Avenacy's Metoclopramide Injection, USP is available in 10 mg/2 mL (5 mg per mL) single-dose vials. In line with Avenacy’s mission to champion patient safety and streamline patient care, Metoclopramide Injection, USP will feature the Company’s highly differentiated packaging and labeling to support accurate medication selection.
Avenacy will begin shipping Metoclopramide Injection, USP to wholesale partners this week. The Company is supported by a global network of development and contract manufacturing partners that have undergone successful FDA inspections based on cGMP-standards.
Metoclopramide Injection, USP had U.S. sales of approximately $12.7 million for the twelve months ending in June 2023.1
Approved Indications:
Diabetic Gastroparesis (Diabetic Gastric Stasis)
Metoclopramide Injection, USP (metoclopramide hydrochloride, USP) is indicated for the relief of symptoms associated with acute and recurrent diabetic gastric stasis.
The Prevention of Nausea and Vomiting Associated with Emetogenic Cancer Chemotherapy
Metoclopramide Injection, USP is indicated for the prophylaxis of vomiting associated with emetogenic cancer chemotherapy.
The Prevention of Postoperative Nausea and Vomiting
Metoclopramide Injection, USP is indicated for the prophylaxis of postoperative nausea and vomiting in those circumstances where nasogastric suction is undesirable.
Small Bowel Intubation
Metoclopramide Injection, USP may be used to facilitate small bowel intubation in adults and pediatric patients in whom the tube does not pass the pylorus with conventional maneuvers.
Radiological Examination
Metoclopramide Injection, USP may be used to stimulate gastric emptying and intestinal transit of barium in cases where delayed emptying interferes with radiological examination of the stomach and/or small intestine.
WARNING: TARDIVE DYSKINESIA
Treatment with metoclopramide can cause tardive dyskinesia, a serious movement disorder that is often irreversible. The risk of developing tardive dyskinesia increases with duration of treatment and total cumulative dose.
Metoclopramide therapy should be discontinued in patients who develop signs or symptoms of tardive dyskinesia. There is no known treatment for tardive dyskinesia. In some patients, symptoms may lessen or resolve after metoclopramide treatment is stopped.
Treatment with metoclopramide for longer than 12 weeks should be avoided in all but rare cases where therapeutic benefit is thought to outweigh the risk of developing tardive dyskinesia (see WARNINGS).
Please see link for Full Prescribing Information including the Boxed Warning.
Reglan® is a registered trademark of ANI Pharmaceuticals, Inc.
1Source: IQVIA
About Avenacy
Avenacy is a U.S.-based specialty pharmaceutical company focused on supplying critical injectable medications used to treat patients in various medically supervised settings, from acute care hospitals to outpatient clinics and physician offices. Through a rigorous and optimized selection process, the Company is building out a pipeline of high-quality FDA approved injectable products in order to ensure a resilient portfolio that can meet the needs of today’s dynamic drug supply chain. With an experienced team, commitment to quality and reliability, and product offerings intended to facilitate safe and efficient patient care, Avenacy strives to be a trusted partner for essential medications.
Avenacy was launched in 2023 and is headquartered in Schaumburg, IL. For more information, please visit http://www.avenacy.com/.
View source version on businesswire.com: https://www.businesswire.com/news/home/20240916846646/en/
Contacts
Media
FTI Consulting
Avenacy@fticonsulting.com




